
- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
BABIO Strengthens Global Diagnostic Supply with Advanced Non-Inactivated Virus Transport Kit
2025-10-18
BABIO Strengthens Global Diagnostic Supply with Advanced Non-Inactivated Virus Transport Kit
Jinan BABIO Biotechnology Co., Ltd. (BABIO), a leading Chinese manufacturer of medical diagnostic reagents, proudly launches its Virus Transport Kit (Non-inactivated) — an essential tool for safe and efficient viral specimen collection and transport across global healthcare and research settings.
Designed with precision and reliability in mind, BABIO’s Virus Transport Medium (VTM) ensures sample integrity during transport, storage, and testing. The formulation is based on an enhanced Hank’s solution, enriched with multiple antibiotics to inhibit bacterial and fungal contamination — ensuring accurate downstream PCR, molecular testing, and viral isolation.
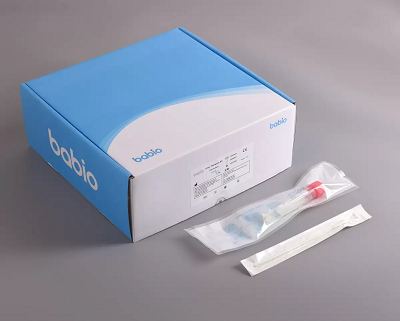
Each kit includes high-quality flocked swabs and shatterproof transport tubes, available in multiple specifications (1–6 ml) and package sizes (20 – 500 pcs) to meet diverse laboratory needs. The DNase/RNase-free conical tubes also enable centrifugation, improving workflow efficiency.
As a trusted diagnostic supplier since 2003, BABIO combines high-volume manufacturing capacity (100,000 kits/day) with CE-certified quality to support global distribution — from hospitals and clinical labs to epidemic control centers. The company’s non-inactivated VTM is already recognized across Europe, the Americas, Africa, and Southeast Asia for its stability, flexibility, and cost-effectiveness.
To explore more about BABIO’s full range of virus transport media, diagnostic reagents, and OEM solutions, visit:
https://www.babiocorp.com
#BABIO #VirusTransportKit #VTM #SampleCollection #Diagnostics #InVitroTesting #GlobalHealth #LabSupplies #MolecularDiagnostics


