
- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
BABIO Secures U.S. FDA 510(k) Clearance for Its Virus Transport Kit (Non-inactivating)
2025-10-10
BABIO Secures U.S. FDA 510(k) Clearance for Its Virus Transport Kit (Non-inactivating)
Jinan, China – October 2025 – Jinan Babio Biotechnology Co., Ltd. (BABIO) proudly announces that its Babio® Virus Transport Kit (Non-inactivating) has officially received FDA 510(k) clearance (K250205). This certification marks a significant milestone for BABIO, reaffirming its commitment to global quality, safety, and innovation in clinical diagnostics and microbiological transport systems.
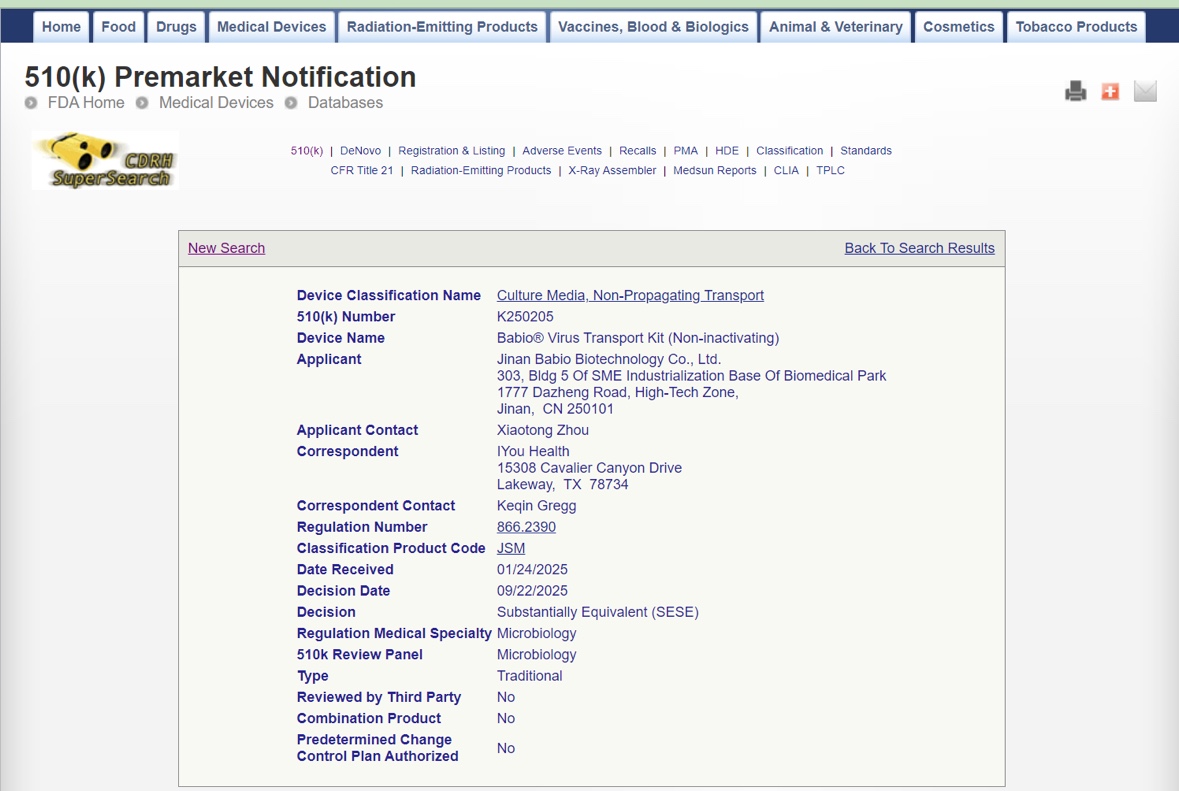
The FDA’s 510(k) clearance authorizes the Babio® Virus Transport Kit as substantially equivalent to legally marketed devices in the United States, validating its compliance with U.S. regulatory standards for medical devices. This achievement demonstrates BABIO’s strong R&D capabilities and manufacturing excellence, further strengthening its global competitiveness in the virus transport and specimen collection market.
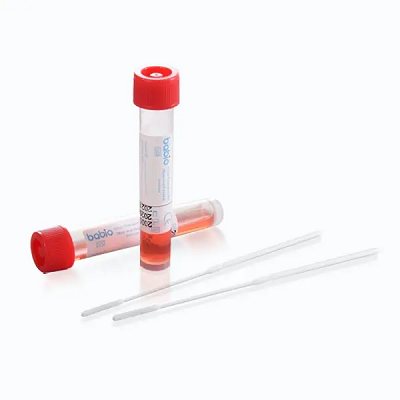
The Babio® Virus Transport Kit (Non-inactivating) is designed for the collection and safe transport of clinical specimens containing viruses. It maintains sample integrity for downstream testing such as RT-PCR, viral culture, and molecular diagnostics, making it suitable for hospitals, laboratories, and public health institutions worldwide.

BABIO, a leading Chinese manufacturer of diagnostic reagents, transport media, and culture media, continues to expand its presence in Europe, the United States, Africa, and Southeast Asia, providing trusted solutions that meet international standards.
For more information about BABIO’s certified products and diagnostic innovations, please visit: https://www.babiocorp.com
#BABIO #FDA510k #VirusTransportKit #MedicalDevices #Diagnostics #BiotechChina #GlobalHealthcare #ClinicalDiagnostics #Microbiology


