
- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
BABIO Introduces Influenza A/B Antigen Rapid Detection Kit
BABIO Introduces Influenza A/B Antigen Rapid Detection Kit
Seasonal Influenza A and B viruses remain a major public health threat worldwide, causing millions of respiratory infections each year. To support fast and reliable influenza diagnostics, BABIO, a leading Chinese manufacturer of in vitro diagnostic solutions, now offers the Influenza A/B Antigen Detection Kit (Colloidal Gold Method).
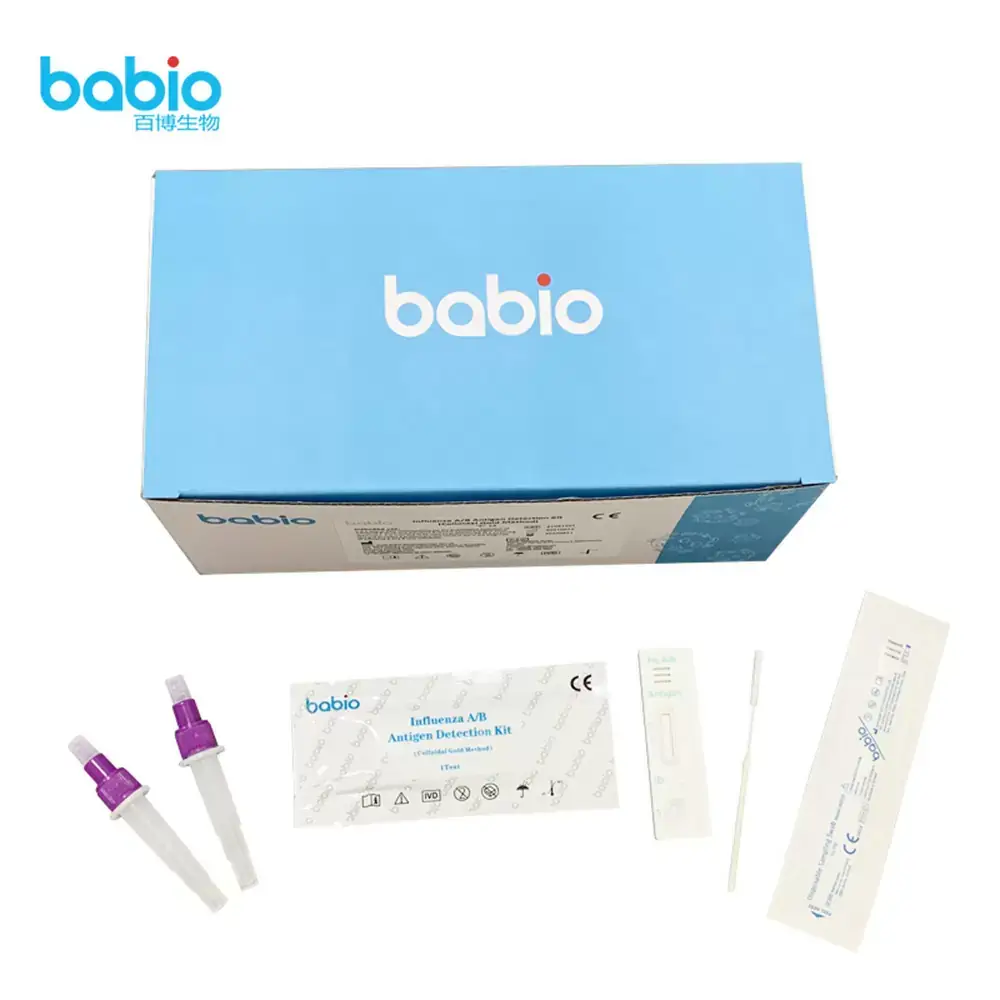
Rapid Dual Detection of Influenza A and B
This lateral flow immunoassay enables the qualitative detection of Influenza A and B antigens in patient samples. The kit features:
-
Dual Test Lines (T1 & T2) – Separate detection of Influenza A and Influenza B antigens.
-
Clear Results in 15 Minutes – Easy-to-read colored lines for fast decision-making.
-
Built-In Control Line (C Line) – Ensures test validity with every run.
-
Simple Workflow – Uses extraction buffer and dropper-based application, no lab equipment required.
Global Relevance in Outbreak Control
With influenza outbreaks reported in Europe, North America, Africa, and Southeast Asia, rapid antigen detection plays a critical role in clinical diagnosis, infection control, and outbreak surveillance. BABIO’s Influenza A/B Antigen Detection Kit offers healthcare providers a reliable, point-of-care tool to differentiate between Influenza A and B infections.
About BABIO
Founded in 2003, BABIO has grown into one of China’s well-recognized manufacturers of diagnostic reagents, rapid tests, and microbial detection kits. With ISO-certified facilities and international distribution, BABIO continues to provide innovative, cost-effective solutions for global healthcare needs.
👉 Learn more about the Influenza A/B Antigen Detection Kit at: https://www.babiocorp.com.
#Influenza #FluTest #RapidTestKit #InfectiousDisease #Diagnostics #BABIO #RespiratoryHealth #PointOfCare


